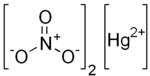
Mercury(II) nitrate
|
|
| Names | |
|---|---|
|
IUPAC names
Mercury dinitrate
Mercury(II) nitrat |
|
| Other names
Mercuric nitrate
|
|
| Identifiers | |
|
|
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.126 |
| EC Number |
|
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1625 |
|
CompTox Dashboard (EPA)
|
|
|
|
|
|
| Properties | |
| Hg(NO3)2 | |
| Molar mass | 324.60 g/mol (anhydrous) |
| Appearance | colorless crystals or white powder |
| Odor | sharp |
| Density | 4.3 g/cm3 (monohydrate) |
| Melting point | 79 °C (174 °F; 352 K) (monohydrate) |
| soluble | |
| Solubility | soluble in nitric acid, acetone, ammonia insoluble in ethanol |
| −74.0·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
   
|
|
| Danger | |
| H272, H300, H310, H330, H373, H410 | |
| NFPA 704 (fire diamond) | |
| Flash point | Nonflammable |
| Safety data sheet (SDS) | ICSC 0980 |
| Related compounds | |
|
Other anions
|
Mercury(II) sulfate Mercury(II) chloride |
|
Other cations
|
Zinc nitrate Cadmium nitrate |
|
Related compounds
|
Mercury(I) nitrate |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
|
|
Mercury(II) nitrate is an inorganic compound with the formula Hg(NO3)2.xH2O. These colorless or white soluble crystalline salts are occasionally used as a reagent. It is made by treating mercury with hot concentrated nitric acid. Neither anhydrous nor monohydrate has been confirmed by X-ray crystallography. The anhydrous material is more widely used.
Uses
Mercuric nitrate has been used in mercuration of ketones. Mercuric nitrate was formerly used in carroting felt for hats.
Health information
Mercury compounds are highly toxic. The use of this compound by hatters and the subsequent mercury poisoning of said hatters is a common theory of where the phrase "mad as a hatter" came from.
